Part 8 - Aerobic Biosynthesis
==================================================
v Anaerobic
ande aerobic biosynthesis
Yeasts do not need oxygen to convert glucose to ethanol
or to synthesize glycerols, saturated fatty acids and proteins from amino
acids. However, oxygen is needed for :
-
biosynthesis of ATP in Electron Transport Chain (see Part 3),
-
biosynthesis of unsaturated fatty acids (UFA), and
-
biosynthesis of ergosterol.
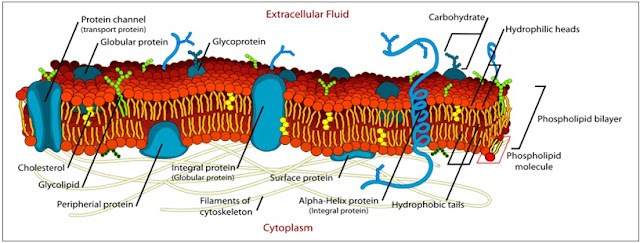
v Phospholipids bilayer
A yeast cell membrane is constructed of phospholipids. A phospholipid
consists of a phospho-head and two fatty acid-tails (lipid = fat ). The
phospho-head is hydrophilic (love water); the fatty acid-tails are hydrophobic
(fear of water).
For this reason, the yeast cell membrane is formed by a
"tail-to-tail” bilayer of phospholipids. The phospholipids float against
each other. They are not fixed to each other. They allow proteins and enzymes
to move freely between them. At low temperature they become tightly close and
at high temperature they drift loosely. The yeast cell membrane is a fluid
mosaic model.
v Proteins, Carbohydrate & Sterol
The cell membrane consist of not only phospholipids, but
also many other substances, which can be divided into 3 groups. (1) Different proteins,
for different functions, synthesized from different amino acids.
(2) Carbohydrate
is a carbon where water is included (H-C-OH). Carbohydrate attached to protein
or phospholipid is called glycoprotein resp. glycolipid.
(3) Sterol, which is called
cholesterol in animal and ergosterol in yeast.
v Unsaturated fatty acids (UFA) & medium chain
fatty acids (MCFA)
The yeast cell membrane is a fluid mosaic model.The fluidity
of the yeast cell membrane is considerably reduced by low temperature and high
ethanol concentration. The phospholipids go tightly against each other. This
can prevent cellular transport systems from functioning correctly. Therefore,
during alcoholic fermentation yeasts must adapt the membrane fluidity to the
changing environmental conditions. They can do that by synthesizing unsaturated
fatty acids (UFA) or medium chain fatty acids (MCFA). They both have a lower
melting point and more flexibility, and therefore they could modulate the
membrane fluidity. Only for UFA, oxygen is required to dehydrogenate at a defined
position in fatty acids
(-CH2-CH2- + O à -CH=CH- + H2O).
The enzyme, desaturase OLE1, catalyses this dehydrogenation, and is activated by low
temperatures and the presence of oxygen.
v Ergosterol
The yeast can also modulate the membrane fluidity by increasing
its proportion of ergosterol. Ergosterol is a fatty substance that is located
between the fatty acid tails in the membrane. It ensures that the phospholipids are
not too close together at low temperature and not too far apart at high
temperature. Ergosterols (like fatty acids) are synthesized from acetyl-CoA by
the mevalonate pathway. It is a very complicated pathway of about 30 steps. The
key step is, without any doubt, the reaction catalysed by squalene
monooxygenase which uses oxygen as a substrate to transform squalene into
squalene 2.3-expoide. Without oxygen, the ergosterol synthesis will stop there.
v Membrane
fluidity adaptation during fermentation
l
Red wines are fermented at relatively high temperatures (28-30oC)
and are aerated in order to enhance colourextraction. High temperatures cause
excessive fluidity which can alter the organization and the dynamic properties
of the membrane. The increasing ethanol concentration creates a new aggressive
environment. Under these conditions, the yeast must increase their proportion
of UFA and ergosterols to compensate for this effect and consequently enhance
their tolerance to ethanol. These changes can be done without problems because
oxygen is introduced during the racking process.
l
White wines are made at low temperatures (14-18oC) and without
aeration to conserve aromas. The low temperature and the increasing ethanol
concentration prompt the yeasts to adapt their membrane fluidity by increasing
the proportion of UFA and ergosterols. However, these can not go on when the
oxygen is running out. The yeasts need to use another strategy to fluidize
their membranes and the only possibility is incorporating medium chain fatty
acids (MCFA).
l
Long-chain fatty acids (LCFA) and medium-chain
fatty acids (MCFA) can form esters with
alcohols. The volatility of the esters (boiling point) is dependant on the
length of the compound: generally the longer the chain, the less volatile. As
we already know esters contribute aromas to wines, and these aromas will
completely be gone within 1 or 2 years by hydrolysis. That explains why esters
are of more significance to the young white wines than to the reds.
P.S.
Next post we'll take a look at the acetic acid, the main
volatile acid in wine.